Details of the Drug
General Information of Drug (ID: DMLHOA0)
| Drug Name |
Xatral
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alfetim; Alfoten; Urion; Uroxatral; Alfuzosin HCl; Alfuzosin Hydrochloride; Alfuzosin hydrochloride [USAN]; Xatral OD; Xatral Retard; Xatral SR; Xatral XL; SL 77 499-10; SL 77499-10; SL-7749910; Uroxatral, Alfuzosin hydrochloride; Alfuzosin hydrochloride (JAN/USAN); SL-77499-10; SL-77.0499-10; N-[3-[4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro-2-furancarboxamide hydrochloride; N-[3-[(4-amino-6,7-dimethoxyquinazolin-2-yl)-methylamino]propyl]oxolane-2-carboxamide hydrochloride; N-{3-[(4-amino-6,7-dimethoxychinazolin-2-yl)(methyl)amino]propyl}tetrahydrofuran-2-carboxamidhydrochlorid; (+-)-N-(3-((4-Amino-6,7-dimethoxy-2-quinazolinyl)methylamino)propyl)tetrahydro-2-furamide monohydrochloride
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
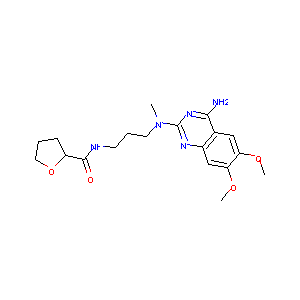 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 425.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Benign prostatic hyperplasia | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | GA90 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
